- ID Number 14265
- Aug 08, 2023
- 202
Ministry of Health & Prevention Confirms the Safety and Good Quality of Brufen, an Anti-inflammatory, Pain Killer and Antipyretic for Children
The Ministry of Health and Prevention (MOHAP) recently confirmed the high quality and safety of Brufen, an anti-inflammatory, analgesic and antipyretic medicine registered in the UAE for use in children. There are no warnings against this medicine.
H.E. Dr. Amin Hussein Al Amiri, the Ministry's Assistant Undersecretary for Public Health Policy and Licensing and Chairman for Supreme National Pharmacovigilance committee, kept in mind that rumors circulated on social media sites relating to the visibility of aluminum bits in Brufen are incorrect. The Ministry has actually not released any type of warnings about making use of Brufen, and has actually not obtained any kind of alerts from the Food and Drug Administration (FDA), the European Medicines Authority (EMA), or the Australian Drug Authority. The Ministry has actually also exerted to communicate with the manufacturer of the drug, who declared that these insurance claims are incorrect.
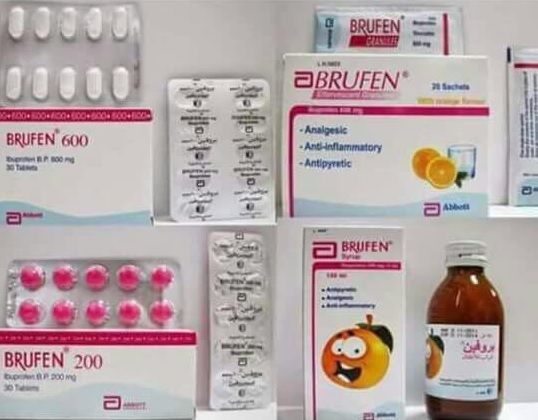
Brufen, a non-steroidal anti-inflammatory drug, is understood medically as advil and is sold over-the-counter as an anti-inflammatory and pain killer, it could be made use of for the pain in the teeth, joints, and for headaches. It is also antipyretic. Brufen is available as syrup for children and in tablet kind in doses of 200mg, 400mg, and 600mg.
The discontinued use of drugs by patients, as a result of misleading information, can cause serious health implications.
H.E. Al Amiri kept in mind that the flow of these unproven rumors can cause panic in the community, and particularly for clients that either stop using important medications or customize their dosages without consulting with their medical care professional. These rumors can lower individual's self-confidence in the quality of medicines, and the integrity of the health systems in the UAE. Al Amiri also noted that the Ministry conducts routine evaluations of medicines and checks them in Quality Control lab based at the Dubai Biotech Research Park, to ensure the quality and security of the medications readily available in the UAE.
Community members are urged to contact the Ministry of Health & Prevention or relevant health authorities if they receive rumors regarding medicines.
The Assistant Undersecretary urged participants of the community to refrain from thinking reports published on social networks, and to speak to the Ministry of Health & Prevention or relevant health authorities to attain any type of details pertaining to medical topics: medications, clinical warnings and anything else concerning medicines and medical devices in the UAE.
He further emphasized the importance of increasing community understanding on incorrect medical rumors, which supports the Ministry's efforts to offer the finest health solutions. H.E. Al Amiri noted that, need to participants of the community dream to confirm any info relating to the health products signed up in the UAE, they rate to speak to the Ministry using email at pv@moh.gov.ae. The public are also welcomed to contact the Ministry by means of its Tamini service at www. moh.gov.ae/ en/Services/Pages/ TaminiService.aspx.

Making History: ASPIRE to Launch Inaugural ‘Abu Dh...
- Apr 23, 2024

ENOC Group partners with Loyyal to enhance ‘YES’ r...
- Apr 23, 2024

Septuagenarian Visitor's Life Saved at Kuwait Hosp...
- Apr 23, 2024












